Popcorn is a beloved snack enjoyed by people across the world. When popcorn kernels are heated, they expand and pop, turning into light and fluffy popcorn.
This transformation occurs through a process that can be classified as either endothermic or exothermic. But which one accurately describes the chemical reactions behind microwave popcorn? Let’s take a closer look at the science behind this popular snack.
What Happens When Popcorn Pops?

When popcorn kernels are heated, the water inside the kernel turns into steam. This builds up pressure within the kernel until the hard outer hull ruptures. The starch inside the kernel then rapidly expands, turning the kernel inside out and creating the iconic popped popcorn shape.
As the starch expands, the strong kernels transform into a foam-like structure. This puffed up interior is what gives popped popcorn its light, airy texture.
So in summary:
- Heating turns water into pressurized steam
- Steam builds up pressure within kernel
- Hard outer hull ruptures
- Starch rapidly expands
- Kernel turns inside out
- Foam-like, puffed up interior forms
This sequence of physical and chemical changes during the popping process holds the clues to whether it is endothermic or exothermic.
What is an Endothermic Process?
An endothermic process is one that absorbs heat. Endothermic reactions draw heat in from the surrounding environment. As a result, the surroundings become cooler.
Some examples of endothermic processes include:
- Evaporation of water
- Melting of ice
- Dissolving ammonium nitrate in water
- Chemical reactions that require an input of energy to proceed
During an endothermic process, energy is absorbed in the form of heat. This energy is used to break bonds holding molecules together so that the molecules can rearrange into new configurations.
What is an Exothermic Process?
In contrast, an exothermic process releases heat. Exothermic reactions give off heat to the surroundings, resulting in an increase in temperature.
Some examples of exothermic processes include:
- Burning of wood or fossil fuels
- Respiration in living organisms
- Setting of cement or concrete
- Chemical reactions that release energy as heat
In an exothermic process, bonds holding molecules together are broken, resulting in more stable molecules. The excess energy released in the form of heat.
So in summary:
- Endothermic – absorbs heat
- Exothermic – releases heat
With this background in mind, let’s evaluate popcorn popping to determine if it is endothermic or exothermic.
is popping popcorn a chemical change
The process of popping popcorn definitely involves a chemical change. Kernels of popcorn contain water and starch, with small amounts of protein and fat. The starch is mostly composed of two glucose polymers called amylose and amylopectin. When the kernels are heated, the water inside turns to steam, building up pressure within the kernel. This pressure causes the starch granules to gelatinize, unwinding the starch polymers. The pressure continues building until the kernel ruptures with a “pop.”
The gelatinized starch polymers then reconfigure themselves into a foam structure. This new molecular configuration gives popped popcorn its light, airy texture. The conversion from hard kernel to fluffy popcorn demonstrates that new chemical bonds are formed during popping. So next time you make popcorn on the stove or in the microwave, you can explain to friends and family that you’re witnessing an entertaining example of a chemical change!
Is Popcorn Popping Endothermic or Exothermic?

When analyzing the popcorn popping process, there are two key factors that indicate whether heat is being absorbed or released:
- Phase Change
- Temperature Change
Let’s look at how both of these factors apply to microwave popcorn:
Temperature Change
As the popcorn kernels are heated in the microwave, the temperature rises rapidly. This input of heat provides the energy needed to induce the chemical changes that cause popping.
Once the kernels have fully popped, the temperature starts to decrease again as the heat dissipates into the surroundings.
Because there is an overall increase followed by decrease in temperature, popcorn popping displays characteristics of an endothermic process. The microwaves provide thermal energy that is absorbed by the kernels to fuel the chemical reactions.
Phase Change
The popping process also involves a phase change from liquid water to steam. As the kernels are heated, the water transitions from a liquid to a gas.
Phase changes from liquid to gas are endothermic processes. They require an input of energy to break intermolecular bonds and allow the change between states of matter.
The creation of steam adds further evidence that popcorn popping is endothermic. The input of thermal energy is needed to evaporate the water content within the kernels.
The Role of Pressure
In addition to temperature change and phase change, internal pressure builds up within the kernel as the water evaporates and steam expands.
This increase in pressure leads to the hard outer hull rupturing open. The starch inside then rapidly expands due to the sudden pressure release.
So while pressure plays an important mechanical role in the popping process, it does not indicate whether the reaction is endothermic or exothermic. Pressure builds up as a result of the phase change from heating.
In summary, pressure:
- Builds up from steam formation
- Leads to hull rupture
- Allows rapid starch expansion
- Does not classify reaction type
The absorption of heat for evaporation and the temperature changes remain the key factors showing popcorn popping is an endothermic process.
Analyzing the System Components
To gain further insight, let’s analyze the key components that make up the system of microwaved popcorn:
System Components
- Popcorn kernels
- Microwave oven
- Microwaves
- Popped popcorn
Popcorn kernels start out at lower temperature and in a lower energy state. As they are heated by the microwaves, the kernels absorb thermal energy from the environment, undergoing internal changes that require energy input.
Meanwhile, the microwave oven does not significantly change in temperature. It releases microwaves that are absorbed by the kernels. Overall, the oven gives off energy to heat up the popcorn.
Finally, the popped popcorn ends up at a higher temperature after absorbing microwaves. The kernel components rearrange into a higher energy state.
Tracking the energy and temperature changes in the system provides additional evidence that popcorn popping is an endothermic process. The kernels absorb heat and end up at higher temperature and energy.
Is the Presence of Steam Indicative?

One question that often arises is whether the presence of steam during popping implies an exothermic reaction. Since steam releases energy as it condenses, doesn’t that make the process exothermic overall?
This would be a reasonable hypothesis, however the steam itself is not a sufficient indicator of the reaction type. The creation of steam through evaporation requires heat input. The steam then condenses and releases heat once the popping is complete.
So the cycle goes:
- Heat absorbed to evaporate water
- Steam created Steam condenses and releases heat after popping
- The key takeaway is that steam formation is only possible with an initial input of heat. The endothermic evaporation process outweighs the exothermic condensation.
- Only analyzing the condensation phase can be misleading. The full cycle must be considered, which shows popcorn popping to be endothermic overall.
Exploring the Exothermic Possibilities
Up to this point, we have demonstrated that popcorn popping is an endothermic process due to the heat absorption, temperature changes, and phase changes involved. However, one may wonder if any parts of the reaction are exothermic. Let’s explore further:
. Starch Decomposition
When the kernels are heated, the starch can become decomposed into simpler sugars. Starch is a complex carbohydrate made up of long chains of glucose molecules.
Breaking starch into glucose requires the breaking of covalent bonds, which is an endothermic process requiring energy input.
So starch decomposition itself does not release heat or indicate an exothermic reaction.
. Water-Starch Interactions
As the water evaporates within the kernel, the starch crystallites can absorb the free water molecules. This makes the starch less rigid so that it can expand more easily when heated.
This water absorption process is endothermic, as it requires energy for water to break hydrogen bonds with itself and form new bonds with starch.
. Starch Gelatinization
When heated in water, starch granules swell up and the molecular order is disrupted, forming a gelatinous mass. This gelatinization process is also endothermic.
Heating causes hydrogen bonding between water and starch, requiring an input of energy. Overall, the starch changes are endothermic.
. Maillard Reactions
Heating starch and sugars together can result in Maillard reactions that produce aromas and brown pigments, enhancing flavor.
However, Maillard reactions proceed too slowly at popcorn popping temperatures to contribute significantly. And these reactions require energy input, so they are not exothermic.
In summary, while the popcorn chemistry is complex, there do not appear to be any major exothermic processes involved. The endothermic nature of water evaporation, starch changes, and temperature rises dominate the reaction.
The Crucial Role of Water
One of the key requirements for popcorn popping is the presence of water within the kernel. This water allows steaming and starch gelatinization to occur.
Popcorn kernels typically contain 13-14% moisture. This water content is crucial, as kernels with too little moisture do not pop well. They require water to absorb enough microwave energy to rupture the hull.
As the microwave heating progresses, the water transitions from liquid to vapor, absorbing significant heat in the process. This evaporation keeps the kernel temperature regulated so the starch does not overheat too quickly before the hull pops.
So water plays several important roles:
- Provides pressure for hull rupture through steaming
- Allows starch expansion through gelatinization
- Absorbs heat through evaporation
- Regulates kernel temperature
These mechanisms rely heavily on endothermic processes, further confirming popcorn preparation to require heat input overall.
Does Microwave Heating Change the Reaction?

Up to this point, we have focused on popcorn popping by microwave oven. An important question is whether using microwave heating instead of conventional heating alters the endothermic nature of the process?
Microwaves function by causing polar water molecules within the kernels to oscillate and generate thermal energy through molecular friction. This evenly heats the water throughout the kernels, generating pressure and allowing popping.
However, microwave heating alone does not change the basic chemical pathways involved, which remain centered around endothermic water evaporation. The same starch gelatinization and steam formation occurs.
So whether heated by microwaves, hot air, or oil, the underlying chemistry remains endothermic. Microwave heating provides an efficient means of generating the heat needed to induce the necessary changes.
Evaluating the Entropy Change
In chemistry, endothermic and exothermic processes can also be classified according to the change in entropy that occurs during the reaction. Entropy is a measure of molecular disorder in a system.
For an endothermic process, entropy increases as energy is absorbed. This results in more molecular motion and dispersal of matter and energy.
In contrast, exothermic processes decrease entropy as energy is released. Molecular motion decreases as energy dissipates.
When popcorn kernels pop, the starch and water components become much more disordered. The once tightly arranged kernel transforms into a puffed up foam-like structure high in entropy.
This large increase in entropy provides additional evidence that popcorn popping is an endothermic reaction. The absorption of thermal energy allows more disorder in the system.
How Microwave Ovens Work
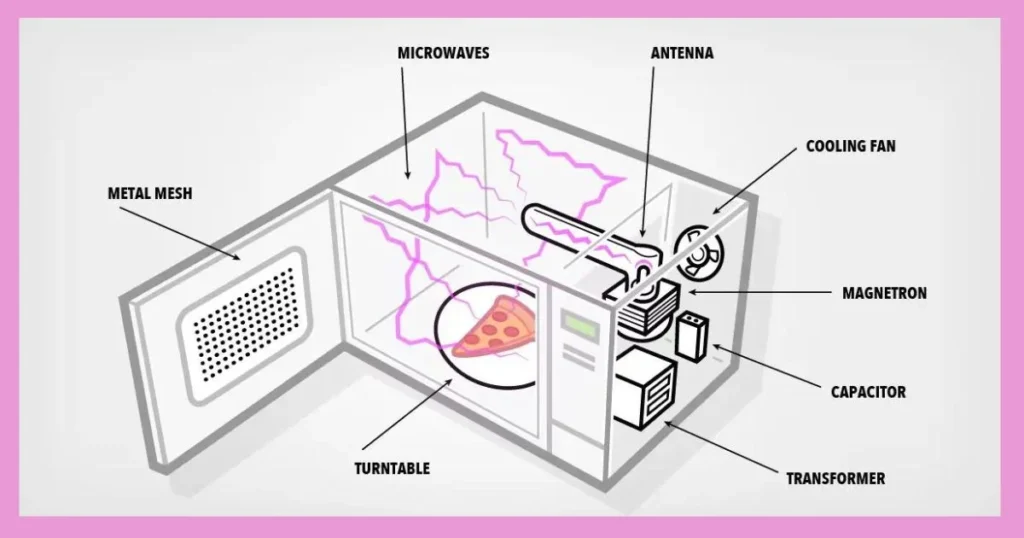
To fully appreciate the endothermic nature of popcorn popping, it helps to understand how microwave ovens function to deliver energy into the kernels through radiation.
Microwave ovens contain a magnetron that generates electromagnetic waves at a frequency of 2.45 GHz. This high frequency radiation is able to penetrate several centimeters into materials containing water and fats.
In popcorn kernels, the microwaves are absorbed by water molecules, causing them to oscillate 4.9 billion times per second. These incredibly rapid vibrations generate friction and heat up the water.
This efficient heating occurs through a process called dielectric heating. Dipoles within the water molecules align themselves with the oscillating electric field. As the field shifts orientation, the molecules flip around, resulting in motion and thermal energy generation.
The microwaves provide the external source of energy needed to add heat into the popcorn kernels and induce popping. This exemplifies the endothermic nature of the process.
How a Microwave Oven Works Summary:
- Magnetron generates 2.45 GHz microwaves
- Microwaves penetrate into food containing water
- Water molecules oscillate 4.9 billion times per second
- Molecular motion creates friction and heats up water
- Dielectric heating transfers microwave energy into thermal energy
Conclusion
In conclusion, popcorn popping is an endothermic process based on the evidence of:
- Heat absorption from surroundings
- Increases in temperature
- Phase changes from liquid water to steam
- Entropy increases as popcorn pops
- Energy required for starch gelatinization
The microwave oven provides the external source of thermal energy needed to drive this endothermic reaction. The microwaves induce rapid water molecule oscillations that heat up the kernel and generate steam and pressure.
While popcorn chemistry is complex, the overriding endothermic factors of heat absorption for evaporation and starch expansion make the net reaction endothermic. This allows the hard kernels to pop into fluffy, delicious popcorn through rapid steam expansion!
Frequently Asked Questions
Here are some frequently asked questions given below:
Does the steam released mean popcorn popping is exothermic?
No, the steam formation requires an initial input of heat to evaporate liquid water. So even though steam releases heat when it condenses, the overall process requiring heating of water is endothermic.
How does microwave heating provide the energy?
Microwaves generate rapid oscillation of polar water molecules, creating molecular friction that heats up the water. This dielectric heating transfers external energy from the microwaves into thermal energy within the kernels.
Why doesn’t popcorn pop when stale?
Popcorn needs a moisture content around 13-14% of the kernel weight to pop properly. When it stale, the moisture level drops too low, preventing the hydration and swelling needed for the starch to expand fully.
Can you pop popcorn without oil in a microwave?
Yes, you can pop plain kernels in a microwave successfully. The oil simply helps distribute heat but is not required for the popping reaction. The microwave provides enough energy for the water to evaporate and pop the kernels.
Does cooking popcorn on the stove make it exothermic?
No, the fundamental endothermic reactions involving water evaporation and starch gelatinization remain the same. Stovetop heating simply transfers thermal energy differently compared to microwaves, but the net result is still an endothermic process.
What if you tried to pop popcorn in a vacuum?
Popping popcorn requires atmospheric pressure so that steam can accumulate and burst the hull. In a vacuum with no pressure, the water would immediately boil off without creating enough force to pop the kernel. So popcorn needs pressure from surrounding air to support the popping mechanism.
Why does steam cause popcorn to pop but steam from boiling water doesn’t?
The hard outer shell of popcorn kernels allows pressure to build internally before rupturing. In an open pot of water, steam can freely escape into the atmosphere and not generate enough pressure to pop loose kernels. The confined space of the kernel is key.
Does popcorn ever stop popping before all kernels pop?
Yes, in some cases the popping can prematurely end before all kernels have popped. This can happen if the initial moisture content was too low or non-uniform. Drier kernels require more heat to pop, so they may not finish popping once the hottest kernels have expanded and cooling begins.
Can you pop popcorn in a regular oven?
Yes, popcorn can be popped in a conventional oven by putting the kernels in a covered pot or brown paper bag. The hot air provides the heat needed for popping, though it may take longer and be harder to control compared to a microwave. Ovens can burn popcorn easily if the heat is too high.
What is the ideal thickness of a popcorn hull?
The hull needs to be thin enough that pressure can rupture it, but thick enough to withstand normal handling without cracking. Around 0.0008 inches provides an optimal balance of strength and rupture ability for most popcorn varieties. Hull thickness can vary and affect popping performance.
which energy transformation pops the corn kernels
When corn kernels are heated, the water inside them turns to steam. This causes pressure to build up inside the kernel until the hard outer shell bursts open with a pop. The heating process turns the kernels’ stored chemical energy into kinetic energy that pops the corn.
